Two novel mollusk short-form ApeC-containing proteins act as pattern recognition proteins for peptidoglycan
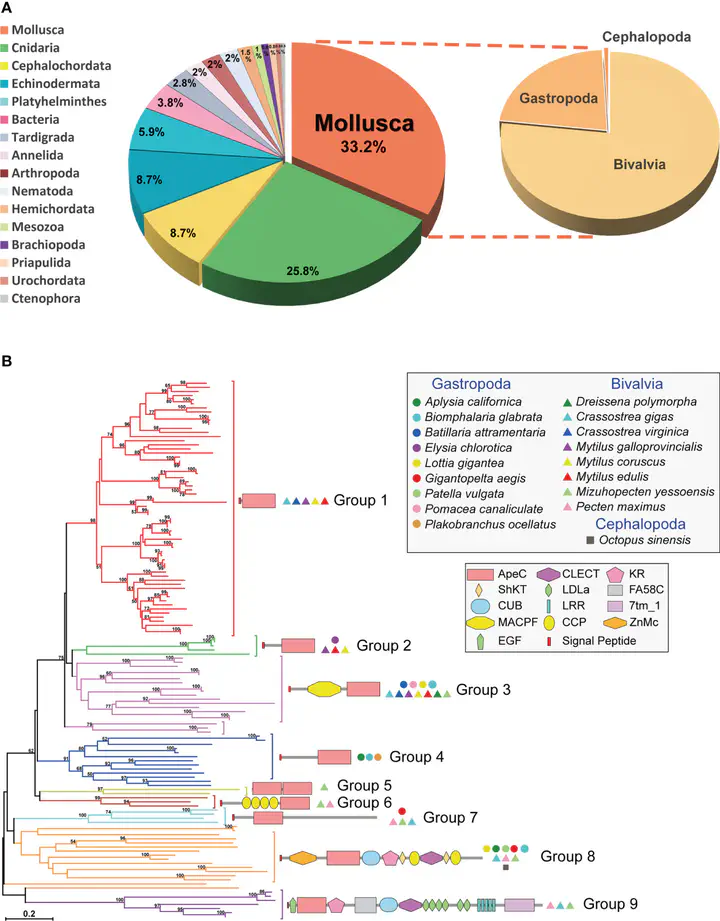 ACPs’ distribution in Mollusk
ACPs’ distribution in Mollusk
Abstract
The Apextrin C-terminal (ApeC) domain is a new protein domain largely specific to aquatic invertebrates. In amphioxus, a short-form ApeC-containing protein (ACP) family is capable of binding peptidoglycan (PGN) and agglutinating bacteria via its ApeC domain. However, the functions of ApeC in other phyla remain unknown. Here we examined 130 ACPs from gastropods and bivalves, the first and second biggest mollusk classes. They were classified into nine groups based on their phylogenetics and architectures, including three groups of short-form ACPs, one group of apextrins and two groups of ACPs of complex architectures. No groups have orthologs in other phyla and only four groups have members in both gastropods and bivalves, suggesting that mollusk ACPs are highly diversified. We selected one bivalve ACP (CgACP1; from the oyster Crossostrea gigas) and one gastropod ACP (BgACP1; from the snail Biomphalaria glabrata) for functional experiments. Both are highly-expressed, secreted short-form ACPs and hence comparable to the amphioxus ACPs previously reported. We found that recombinant CgACP1 and BgACP1 bound with yeasts and several bacteria with different affinities. They also agglutinated these microbes, but showed no inhibiting or killing effects. Further analyses show that both ACPs had high affinities to the Lys-type PGN from S. aureus but weak or no affinities to the DAP-type PGN from Bacillus subtilis. Both recombinant ACPs displayed weak or no affinities to other microbial cell wall components, including lipopolysaccharide (LPS), lipoteichoic acid (LTA), zymosan A, chitin, chitosan and cellulose, as well as to several PGN moieties, including muramyl dipeptide (MDP), N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc). Besides, CgACP1 had the highest expression in the gill and could be greatly up-regulated quickly after bacterial challenge. This is reminiscent of the amphioxus ACP1/2 which serve as essential mucus lectins in the gill. Taken together, the current findings from mollusk and amphioxus ACPs suggest several basic common traits for the ApeC domains, including the high affinity to Lys-type PGN, the bacterial binding and agglutinating capacity, and the role as mucus proteins to protect the mucosal surface.